Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:2 Percentile:76.52(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (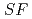
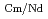 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
Miyagawa, Akihisa*; Hayashi, Naoki*; Kuzure, Yoshiaki*; Takahashi, Takumi*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; et al.
Bulletin of the Chemical Society of Japan, 96(7), p.671 - 676, 2023/07
Times Cited Count:2 Percentile:71.3(Chemistry, Multidisciplinary)We investigated the distribution mechanism of Eu(III) in a single polymer-coated silica particle including nitrilotriacetamide (NTA) extractants known as HONTA and TOD2EHNTA. The present study provides a valuable approach for the evaluation and enhancement of the functionality of "single extractant-impregnated polymer-coated silica particle".
Akuzawa, Tadashi*; Kim, S.-Y.*; Kubota, Masahiko*; Wu, H.*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 331(12), p.5851 - 5858, 2022/12
Times Cited Count:3 Percentile:31.61(Chemistry, Analytical)Sano, Yuichi; Sakamoto, Atsushi; Miyazaki, Yasunori; Watanabe, So; Morita, Keisuke; Emori, Tatsuya; Ban, Yasutoshi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle; Sustainable Energy Beyond the Pandemic (GLOBAL 2022) (Internet), 4 Pages, 2022/07
We developed a hybrid MA(III) recovery process combining MA(III)+Ln(III) co-recovery flowsheet by solvent extraction with TBP and MA(III)/Ln(III) separation flowsheet by simulated moving bed chromatography using HONTA impregnated adsorbents with large particle size porous silica support.
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0502 (Internet), 2 Pages, 2022/04
This report summarizes the results obtained in FY2020 at the Electron Linac Facility of the University of Tokyo. The radiolysis process of  -hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
-hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
Sakamoto, Atsushi; Kibe, Satoshi*; Kawanobe, Kazunori*; Fujisaku, Kazuhiko*; Sano, Yuichi; Takeuchi, Masayuki; Suzuki, Hideya*; Tsubata, Yasuhiro; Ban, Yasutoshi; Matsumura, Tatsuro
JAEA-Research 2021-003, 30 Pages, 2021/06
Japan Atomic Energy Agency has been developing a solvent extraction process called SELECT to recover minor actinides (MA) from spent nuclear fuel. In the SELECT process, TDdDGA, HONTA, and ADAAM are used as the extractants for MA + Ln corecovery, MA/Ln separation and Am/Cm separation, respectively. These extractants do not contain phosphorus (P), and consist of carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). In this study, in order to give beneficial information for designing flowsheet, the mass transfer coefficients of Ln between HNO solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO
solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO / TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO
/ TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO / HONTA - n-dodecane system are under 10
/ HONTA - n-dodecane system are under 10 m/s.
m/s.
 -octylnitrilo-triacetamide (HONTA) complexes of americium and europium
-octylnitrilo-triacetamide (HONTA) complexes of americium and europiumToigawa, Tomohiro; Peterman, D. R.*; Meeker, D. S.*; Grimes, T. S.*; Zalupski, P. R.*; Mezyk, S. P.*; Cook, A. R.*; Yamashita, Shinichi*; Kumagai, Yuta; Matsumura, Tatsuro; et al.
Physical Chemistry Chemical Physics, 23(2), p.1343 - 1351, 2021/01
Times Cited Count:14 Percentile:81.5(Chemistry, Physical)The candidate An(III)/Ln(III) separation ligand hexa- -octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent (
-octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent ( -dodecane) radical cation of
-dodecane) radical cation of 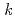 (HONTA + R
(HONTA + R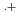 ) = (7.61
) = (7.61  0.82)
0.82)  10
10 M
M s
s obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed
obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed  -dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived (
-dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived ( 100 ns) HONTA radical cation as well as a longer-lived (
100 ns) HONTA radical cation as well as a longer-lived ( s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(7), p.489 - 499, 2019/11
Times Cited Count:15 Percentile:52.81(Chemistry, Multidisciplinary)A continuous counter-current experiment to separate minor actinides (MAs: Am and Cm) was performed with  -hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm
-hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm ) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be
) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be  99% by optimizing separation conditions.
99% by optimizing separation conditions.
Watanabe, So; Suzuki, Hideya; Goto, Ichiro*; Kofuji, Hirohide; Matsumura, Tatsuro
Nihon Ion Kokan Gakkai-Shi, 29(3), p.71 - 75, 2018/09
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Toigawa, Tomohiro; Tsutsui, Nao; Hotoku, Shinobu; Suzuki, Asuka
no journal, ,
no abstracts in English
Toigawa, Tomohiro; Suzuki, Hideya; Ban, Yasutoshi; Ishii, Sho; Matsumura, Tatsuro
no journal, ,
Degradation of extractants and generation of radiolytic products are considered as problem for feasibility of the minor actinides separation process. In this study, degradation amount of hexaoctyl-nitrilotriacetamide (HONTA), which is a candidate extaractant for MA/Ln separation, and its products yields were obtained by using  -rays emitted from Co-60. Our results showed the cleavage sites in HONTA radiolysis and suggest that the mechanism for HONTA radiolysis is differ depending on the absorbed dose.
-rays emitted from Co-60. Our results showed the cleavage sites in HONTA radiolysis and suggest that the mechanism for HONTA radiolysis is differ depending on the absorbed dose.
Toigawa, Tomohiro; Suzuki, Hideya; Ban, Yasutoshi; Ishii, Sho*; Matsumura, Tatsuro
no journal, ,
R&D on partitioning process of the minor actinides (MA) from high level liquid waste (HLLW) were carried out for reduction of the strain on the radioactive wastes disposal. In this study, degradation amount of hexaoctyl-nitrilotriacetamide (HONTA), which is a candidate extaractant for MA/RE separation, and its products yields were obtained by using  -rays emitted from Co-60. Our results showed the cleavage sites in HONTA radiolysis and suggest that the mechanism for HONTA radiolysis is differ depending on the absorbed dose.
-rays emitted from Co-60. Our results showed the cleavage sites in HONTA radiolysis and suggest that the mechanism for HONTA radiolysis is differ depending on the absorbed dose.
 radiolysis of an extractant for minor actinides, Hexaoctyl-nitrilotriacetamide (HONTA), in dodecane diluent
radiolysis of an extractant for minor actinides, Hexaoctyl-nitrilotriacetamide (HONTA), in dodecane diluentToigawa, Tomohiro; Suzuki, Hideya; Ban, Yasutoshi; Ishii, Sho*; Matsumura, Tatsuro
no journal, ,
Radiolytic stability of an extractant, N,N,N',N',N'',N''-hexaoctyl-nitrilotriacetamide (HONTA), for separation between minor actinides and rare-earth elements were investigated by using  -ray emitting from cobalt-60. Degradation amount of HONTA and its products yields were obtained by gas chromatographic analysis. Our results showed the cleavage sites in HONTA radiolysis and suggested that the radiolysis of HONTA was ruled by different mechanism depending on the absorbed dose.
-ray emitting from cobalt-60. Degradation amount of HONTA and its products yields were obtained by gas chromatographic analysis. Our results showed the cleavage sites in HONTA radiolysis and suggested that the radiolysis of HONTA was ruled by different mechanism depending on the absorbed dose.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; et al.
no journal, ,
To continue the utilization of the nuclear fission energy, the management of the high-level radioactive waste is one of the most important issues to be solved. Partitioning and Transmutation technology is expected to be effective to mitigate the burden of the HLW disposal by reducing the radiological toxicity and heat generation. JAEA has been conducting R&D on the MA separation process to remove of MA from HLW and supply the recovered MA to the transmutation system such as ADS. The MA separation process contains three steps. For An(III)+RE recovery process, we developed TDdDGA which has very high performance to recover of MA from high level waste. HONTA and ADAAM were developed for An(III)/RE separation process and Am/Cm separation process respectively. All extractants satisfy CHON principle to minimization of the secondary waste from the process. The separation performances of the flowsheets were evaluated by continuous extraction tests using simulated and genuine high level liquid waste.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; et al.
no journal, ,
PUREX process was established for industrial scale reprocessing plant. TRUEX and the 4 group separation were developed for partitioning of minor actinides from HLW, and demonstrated using genuine HLW. Although the extractants for the processes have excellent performance, the molecules contain phosphorus which could be cause for the secondary waste from the solvent extraction processes. To minimize the radioactive waste, we have conducted research and development of the new reprocessing and MA separation processes using innovative extractants in accord with CHON principle. The extractants for reprocessing process are monoamides as alternative extractants for TBP. For An(III)+RE recovery process, we developed TDdDGA. HONTA and ADAAM were developed for An(III)/RE separation process and Am/Cm separation process respectively. The separation performances of the flowsheets were evaluated by continuous extraction tests using simulated and genuine spent fuel and high level liquid waste.
Matsumura, Tatsuro; Ban, Yasutoshi; Hotoku, Shinobu; Suzuki, Hideya; Tsubata, Yasuhiro; Tsutsui, Nao; Suzuki, Asuka
no journal, ,
 -radiolysis of MA separation agent of HONTA on extraction performance
-radiolysis of MA separation agent of HONTA on extraction performanceToigawa, Tomohiro; Suzuki, Hideya; Ban, Yasutoshi; Ishii, Sho*; Matsumura, Tatsuro
no journal, ,
To evaluate the radiolytic stability of a novel tetradentate extractant of N, N, N', N', N", N"-hexaoctyl-nitrilotriacetamide (HONTA), batch extractions of lanthanide (Ln) elements were performed by using  -irradiated HONTA extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no difference between Ln could be observed. These indicate that the degradation products of HONTA did not act as Ln extractants, and the decay of Ln extraction were caused by degradation of HONTA.
-irradiated HONTA extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no difference between Ln could be observed. These indicate that the degradation products of HONTA did not act as Ln extractants, and the decay of Ln extraction were caused by degradation of HONTA.
 -radiolysis of a minor actinide extractnt on lanthanides extraction
-radiolysis of a minor actinide extractnt on lanthanides extractionToigawa, Tomohiro; Suzuki, Hideya; Ishii, Sho*; Tsutsui, Nao; Ban, Yasutoshi; Matsumura, Tatsuro
no journal, ,
To evaluate the radiolytic stability of an extractant agents for minor actinides such as N, N, N', N', N", N"-hexaoctyl-nitrilotriacetamide (HONTA), batch extractions of lanthanide (Ln) elements were performed by using  -irradiated extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no initial decrease could be observed.
-irradiated extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no initial decrease could be observed.
Suzuki, Hideya; Tsubata, Yasuhiro; Kurosawa, Tatsuya*; Kawasaki, Tomohiro*; Shibata, Mitsunobu*; Matsumura, Tatsuro
no journal, ,
The Japan Atomic Energy Agency is engaged in research and development of the "SELECT process" which is a separation technology for recovering actinides from spent fuel. A highly practical reagent, called HONTA, was developed. In the present study, HONTA was tested for the mutual separation of MA and RE.
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Matsumura, Tatsuro
no journal, ,
The radiolytic degradation of amidic extractants excepted to be used in the minor actinide sepation were observed by using the pulse radiolysis system in The University of Tokyo. It was found that a intermediate specie for degradation was generated not only by direct ionization of the extractant but also derived from the diluent ionization.